Professor Juleen R. Zierath has spent more than 30 years investigating how exercise affects muscle function and insulin resistance. Her discoveries have revealed the profound therapeutic potential of exercise, especially for people with diabetes, who struggle to regulate their blood sugar. Her efforts are now being recognised with the 2024 EASD–Novo Nordisk Foundation Diabetes Prize for Excellence.
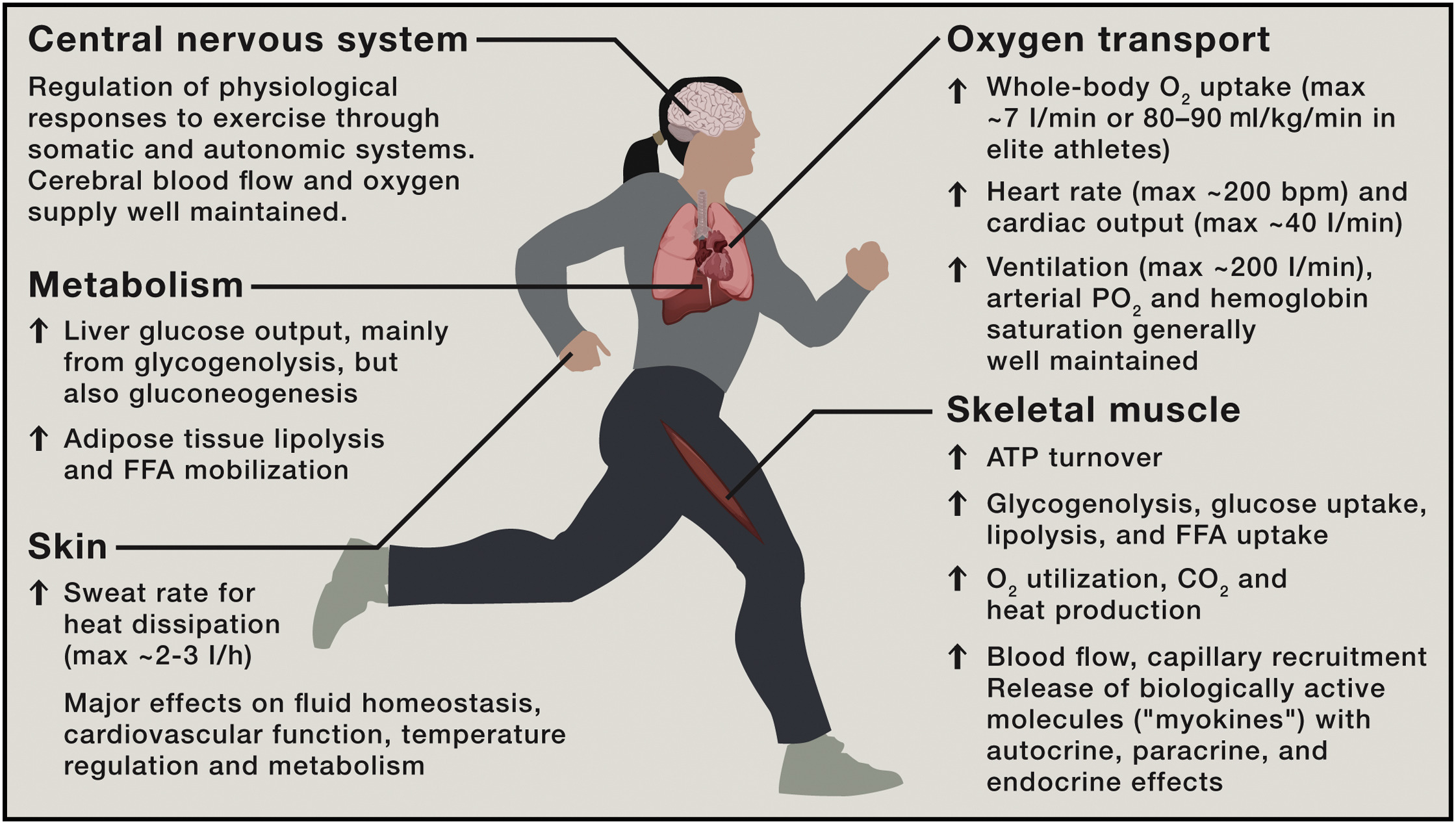
From the moment we start to exercise, our bodies ignite with energy. You breathe faster and heart rate increases. Like a finely tuned engine, you shift up through the gears. Blood surges through your body, delivering the oxygen and nutrients your muscles need to keep up the pace. When you stop, your heart rate falls and your body cools down. But your muscles are still hard at work.
“At a microscopic level, exercise triggers immediate cellular changes. Within minutes to hours after exercising, certain genes become more active. After decades of research, we are now beginning to understand how and why exercise works like medicine and how to time it optimally for both people with metabolic conditions such as diabetes and those without,” explains Juleen R. Zierath, Professor of Clinical Integrative Physiology at Karolinska Institutet in Stockholm, Sweden and Professor and Executive Director at the Novo Nordisk Foundation Center for Basic Metabolic Research at the University of Copenhagen in Denmark.
For more than years, Juleen R. Zierath’s research has shed light on how muscles respond to exercise, how they struggle in conditions such as obesity and type 2 diabetes and why they become insulin resistant. Blending experimental and clinical insights, her research has made significant strides in understanding muscle plasticity.
“Already as a child, I was interested in human health. I was an active athlete and was fascinated by exercise, but I never thought about going to medical school. I was a first-generation college student. No one in my family had ever gone to college before me, so I did not have a lot of role models to help me figure out what I wanted to study.”
When she was about 10 years old, her beloved grandfather had coronary artery bypass surgery. He was 65 years old and just about to retire. The surgery was very new at the time.
“This was the early days of coronary artery bypass surgery in Milwaukee. The big thing they told him after the surgery, because he had been a carpenter and a contractor with a physical job, was to exercise and watch his diet.”
Her grandparents changed their diet and went walking every day. It worked, and he lived to be 95 years old.
“I think that really affected me. Not to say that I think exercise cured him. He clearly had genetics that made him susceptible to atherosclerosis, but I could see how he followed that advice, and it inspired me to try to understand more about how exercise could be used as therapy and prevention for cardiometabolic diseases.”
Exercise as medicine
This experience defined the trajectory of Juleen R. Zierath’s career – to solve the mysteries of exercise and make the findings accessible to benefit everyone.
Juleen R. Zierath compares exercise to a symphony, in which different instruments – organ systems – come together to create beautiful music. In most people, the performance is highly coordinated. During low-intensity efforts, muscles turn to fat for fuel, but when the intensity increases, they smoothly transition to glucose. This remarkable process ensures that the human body uses the most efficient fuel depending on the activity.
“Exercise is the ultimate integrative physiology, meaning that it engages multiple systems and processes within our bodies to work harmoniously,” she says.
In the body’s symphony, skeletal muscles are the primary players in movement and energy use. Adipose tissue stores and releases energy. The liver regulates glucose levels and provides fuel, while the pancreas produces insulin to manage blood sugar. Each organ expresses certain sets of genes, like music sheets transcribed by cellular machinery.
For people with type 2 diabetes, the symphony is disrupted. Their bodies struggle to regulate sugar, causing muscles to tap into the excess glucose in the bloodstream. Exercise is so powerful, however, that it can get the symphony to play in tune despite this underlying issue. This adaptation can help to manage blood sugar levels, turning exercise into a powerful tool for health and balance.
“My mentor, John Holloszy, pioneered the idea that exercise works like medicine. He studied rats with diabetes treated with a compound mimicking human diabetes. These rats had severe insulin resistance, but when their muscles contracted, simulating exercise, their glucose uptake improved significantly.”
A breakthrough in understanding
This breakthrough in 1985 at Washington University School of Medicine demonstrated that exercise could combat insulin resistance, even in severe cases, highlighting its therapeutic potential.
After studying physical education at the University of Wisconsin – River Falls and earning a Master in Exercise Physiology from Ball State University, Juleen R. Zierath joined Holloszy’s lab as a research assistant. Her studies in Holloszy’s lab underscored the complex interplay between diet, exercise and insulin sensitivity and provided insight into optimizing glucose management by modifying lifestyle.
“This really triggered my interest in understanding how muscle cells become insulin resistant and how exercise can help them to remain insulin sensitive. My interest in these questions has grown ever since and has only become more relevant in the context of the growing epidemic of type 2 diabetes and obesity.”
Harriet Wallberg-Henriksson, an influential figure in the field, had returned to Karolinska Institutet in Sweden. Since Juleen R. Zierath was already deeply inspired by Scandinavian physiologists and had known several fellow students studying there, she decided to follow a similar path.
“They spoke highly of the Scandinavian research environment and its collaborative approach, supportive atmosphere, open communication and exchange of ideas. This convinced me to pursue my PhD at the Karolinska Institutet.”
Exploring molecular pathways
Although Karolinska Institutet has been her main base ever since, she moved to Boston after completing her PhD in 1995 for a postdoctoral fellowship with Barbara Kahn at Beth Israel Deaconess, Harvard Medical School. Kahn is a pivotal figure in physiology research, particularly in diabetes and metabolism.
Juleen R. Zierath’s time at Harvard marked a significant career breakthrough due to a groundbreaking study. Scientists knew that insulin and exercise boosted glucose metabolism and gene activity in muscles. But although they understood the precise process through which insulin improved glucose uptake and metabolism, it remained a mystery why exercise had the same effect.
“Our aim was to explore molecular pathways and uncover how exercise enhances metabolic health and combats conditions such as diabetes. Our study revealed that type 2 diabetes involves problems in insulin signalling, including reduced activity of IRS and PI3 kinase enzymes, which are crucial for this process. These defects result in lower glucose uptake by cells, contributing to the high blood sugar levels seen in type 2 diabetes.”
The role of key proteins
When you eat or exercise, insulin signals the transport of glucose into cells for storage and energy use. Signalling molecules, such as IRS and PI3 kinase, aid this process by affecting glucose transporter type 4 (GLUT4), a crucial protein discovered only a few years ago.
“The GLUT4 protein moves glucose from the blood into muscle and fat cells. GLUT4 usually stays inside the cells but moves to the surface when signalled by insulin or during exercise. Exercise boosts GLUT4 movement, making muscles use glucose more efficiently, improving insulin sensitivity and managing blood sugar levels. This understanding is crucial for improving diabetes treatment.”
Back at Karolinska Institutet in Sweden, Juleen R. Zierath started her own group to investigate how exercise boosts metabolism at the cellular level. She collaborated with Anna Krook on experiments on muscle cells from both healthy individuals and those with type 2 diabetes.
“To achieve this in the lab, we subjected the cells to an artificial form of exercise. We used hypoxia, a condition in which the cells do not get enough oxygen, and a chemical called AICAR (5-aminoimidazole-4-carboxamide ribonucleotide), which activates cellular energy sensors, to mimic the effects of exercise.”
The researchers studied a protein called AS160, which plays a crucial role in moving the key glucose gatekeeper GLUT4. For people with type 2 diabetes, insulin’s effect on AS160 was impaired. However, exercise was able to activate this protein, enhancing glucose uptake.
“Understanding these modifications is essential for developing better strategies to control metabolism and combat diseases such as diabetes. We found that exercise caused various modifications – changes that significantly impacted metabolism.”
A dual action of exercise
John Holloszy’s groundbreaking work in the 1980s demonstrated that one week of exercise improved glucose tolerance among men with type 2 diabetes, enhancing insulin sensitivity. Inspired by this work, Juleen R. Zierath and co-workers now tried to move the exercise treatment from the lab to the clinic to better understand the responsible mechanisms.
“Together with John Nolan and Donald Gorman, we found that exercise improved insulin-stimulated glucose uptake among men with obesity or type 2 diabetes. After seven days of training, glucose uptake further enhanced due to an increased abundance of GLUT4,” explains Juleen R. Zierath.
After exercise, there is a quick but temporary increase in levels of mRNA, the templates for making proteins, which results in more proteins that enhance metabolism. The research also showed that consistent exercise leads to long-term adaptations, such as improved performance and metabolic efficiency, which demonstrated the profound impact of physical activity on health.
“Exercise acts as medicine to enhance insulin sensitivity. Our clinical studies have shown significant improvements in glucose tolerance and metabolic health among individuals with type 2 diabetes after just one week of regular exercise. With consistent training, we see dual action – acute improvements in glucose uptake and long-term benefits by increasing the abundance of GLUT4 transporters.”
Insights from elite athletes
Until the early 2000s, Juleen R. Zierath primarily studied how exercise affects people with type 2 diabetes. She then began research on elite athletes to understand how muscles and metabolism adapt. Sprinters have muscles optimized for quick energy bursts, whereas marathon runners have muscles for sustained energy use, helping them to run longer and improving their insulin sensitivity.
“We hoped that by understanding how exercise changes muscles, we could use similar methods to manage diabetes. Muscles are highly adaptable and can alter their energy usage based on different needs. This adaptability is regulated by changes in gene activity, which helps to manage how muscles use glucose and fat.”
When we exercise, our muscles adapt in amazing ways. Two key enzymes, 5′ adenosine monophosphate-activated protein kinase (AMPK) and calcineurin, are activated during exercise and play important roles. AMPK helps to burn fat and increases sensitivity to insulin, crucial for managing blood sugar levels. Calcineurin helps our muscle fibres work more like those of endurance athletes, who can run long distances without tiring quickly.
“This research shows just how flexible our muscles are and how they can adjust to different types of exercise. Interestingly, when untrained people exercise, their muscles respond more strongly than those who are already well trained. This means that, for people who have been exercising regularly, their muscles need a bigger push to get the same reaction.”
Understanding genetic and epigenetic changes
Despite the evidence that exercise treats and prevents metabolic diseases, scientists were unsure how it made its effect. Different types of exercise, such as lifting weights, running and high-intensity workouts, have unique benefits. People also have varied responses to exercise, making outcomes hard to predict. Advances in protein analysis have improved the understanding of these mechanisms.
“Understanding how exercise affects our bodies can lead to personalised workout plans and new treatments. Together with Nicolas Pillon, we studied how different types of exercise impact gene expression for many people. For example, aerobic and resistance training show unique changes in muscle genes, affecting fat use, energy production and immunity, revealing exercise’s full impact. There is also an inactivity signature in the muscles.”
Comparing aerobic and resistance training among healthy individuals and those with metabolic issues, they found that exercise improves mitochondrial genes, the energy centres of cells. Aerobic training enhances heart health and energy production, and resistance training builds muscle and strength. Both aid in processing and storing sugar.
“Overall, exercise boosts energy and health for everyone. By studying these data, we can see how people with metabolic problems respond to exercise. Comparing aerobic and resistance training among both healthy people and those with metabolic issues, we found that exercise significantly improves the function of mitochondria, the energy producers in cells.”
The importance of epigenetics
One big question is how our bodies control genes and proteins based on our environment. This happens through epigenetic changes, which alter gene function without changing DNA. These changes can add chemical tags to DNA through methylation, alter DNA’s wrapping around proteins or use small RNA molecules.
“Think of our genes as books in a library, where adding or removing bookmarks can change how often they are read. For people with type 2 diabetes, we found many genes with added bookmarks, making them less active. These changes, linked to mitochondrial function, show how our environment affects gene activity and explains how factors such as diet, stress and exercise impact our genes and metabolism.”
Together with Romain Barrès, Juleen R. Zierath studied how changes in muscle tissue DNA relate to diabetes. They found that people with type 2 diabetes had increased DNA methylation on two genes, including the one encoding PGC-1alpha – important for producing new mitochondria. This extra methylation reduced the gene’s activity, leading to lower PGC-1alpha levels. Blocking methylation prevented these changes, and muscle cells exposed to fat showed similar patterns.
“When we blocked enzymes that add methyl groups to DNA, we stopped a process triggered by fatty acids and inflammation affecting muscle cells. These factors can change the way transcription factors bind to DNA, reducing the activity of genes important for energy use and insulin function, contributing to insulin resistance in type 2 diabetes.”
The scientists also found that people with obesity showed similar DNA changes, which improved after weight-loss surgery. Many of the DNA changes linked to poor glucose control returned to normal, similar to those in healthy individuals. This suggests that obesity or insulin resistance may change DNA markers and that weight loss can reverse it. But what about exercise?
“We found that, after exercise, certain DNA markers on genes related to mitochondria had fewer methyl groups and higher mRNA expression after exercise, unlike among insulin-resistant people. This change boosts metabolism, improving glucose uptake and muscle function. Signals involving calcium might trigger these DNA changes. Similar to how athletes train for performance, exercise is crucial for health improvements, especially for people with diabetes.”
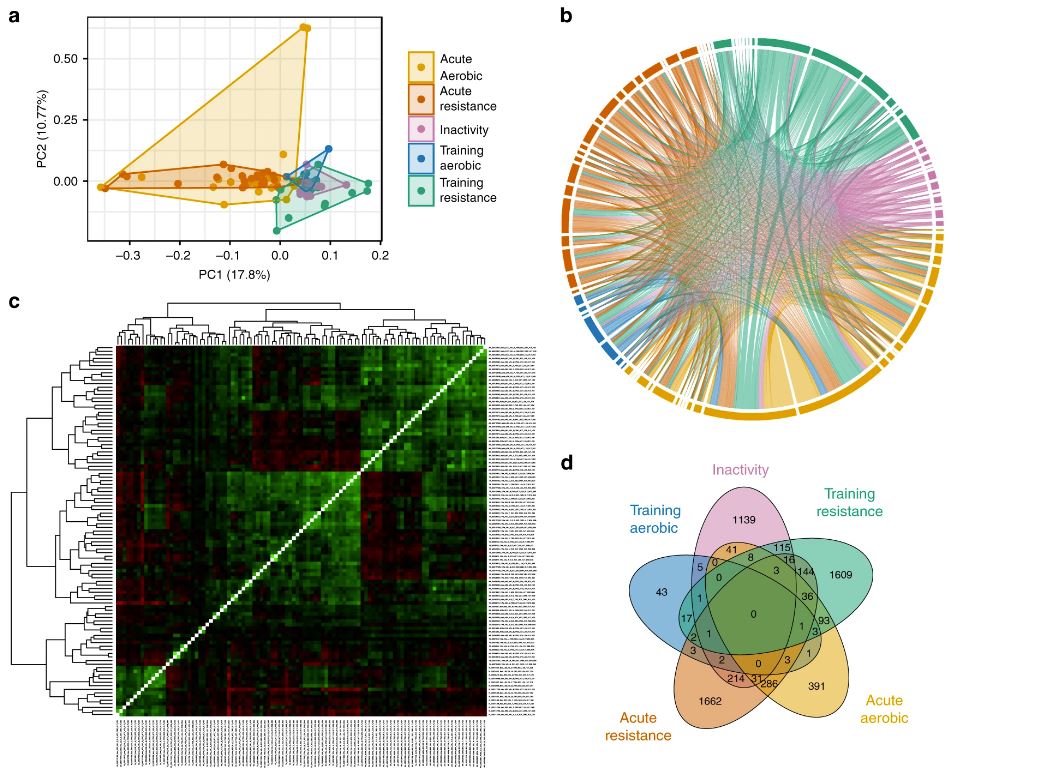
Decoding Exercise Impact: Gene Expression Insights from Acute Workouts, Training, and Inactivity. A comprehensive analysis compares gene expression across exercise, training, and inactivity, revealing distinct biological signatures.
The role of timing in exercise
Juleen R. Zierath is currently exploring how the timing of exercise and meals affects our metabolism. Every cell has a circadian clock, controlled by a central clock in the brain. These clocks in muscles, fat, liver and pancreas are influenced by nutrients, stress and hormones. Understanding this interaction is crucial for improving metabolic health and treating disorders such as diabetes.
They first studied how exercise timing affects metabolism by having rodents exercise at different times of the day to determine the impact on metabolic pathways. In a related clinical study, men with type 2 diabetes performed high-intensity interval training in the morning or afternoon. Blood glucose levels were monitored continuously to determine the influence of exercise timing.
The rodent study showed that exercising early in their active phase significantly boosted metabolism, including sugar and fat breakdown, likely due to activation of the oxygen-sensing protein HIF-1-alpha. Exercise later in the day had minimal effects. In humans, afternoon exercise lowered blood sugar, whereas morning exercise raised it. This indicates that exercise timing may be crucial for metabolic benefits such as improving insulin sensitivity and muscle function.
The research shows that the time of day affects blood sugar control and metabolism. These findings could lead to new ways to use exercise to reset our internal clocks, improve metabolism and enhance health for those with metabolic disorders. Circadian biology may be key for better metabolic health.
The future of exercise and metabolism
Looking ahead, Juleen R. Zierath wants to continue exploring how exercise and our internal clock work together.
“We are investigating whether key players like AMPK, mTOR (mammalian target of rapamycin) or HIF-1-alpha play a role in integrating signals from exercise into this clock machinery and how the time of day may affect our metabolism.”
AMPK acts like a fuel gauge in our cells, mTOR is a key player in cell growth and HIF-1-alpha helps cells to respond to low oxygen levels. As research tools improve, scientists can better grasp the complexity of metabolic regulation.
“We now have tools in our hands to be able to better understand the complexity and different dimensions of metabolism,” Juleen R. Zierath says.
Her team hopes to translate their basic research into practical treatments for metabolic disorders through personalized exercise plans to optimise health, focusing on how different types of exercise impact blood sugar and muscle function in different people. First, many discoveries need to be made.
“To me, it is an evolution. We do not know everything about anything yet, especially in circadian biology, in which diet, nutrients, exercise and even temperature all play a role. The complexity of these interactions makes complete understanding of this biology challenging, but it also makes scientific discovery exciting. That is the amazing thing about science,” she says.
