There is currently great anxiety about and awareness of the scope of antibiotic resistance. Nevertheless, there is little focus on biofilm infections although they are overall more common, are a current and growing problem and also involve antibiotic resistance and tolerance. Biofilms formed by bacteria cause an estimated 60–70% of bacterial infections in hospitals.
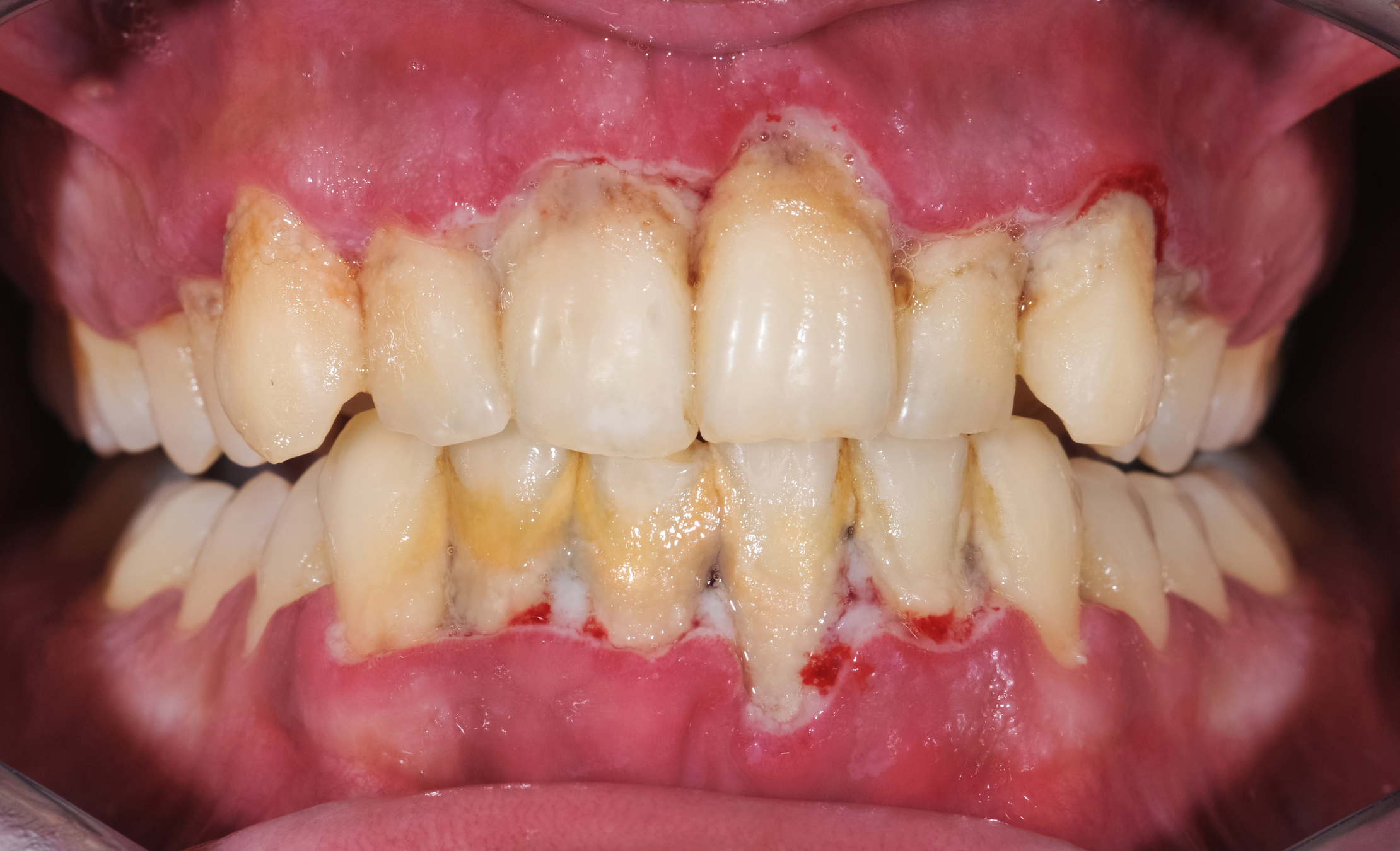
Biofilms may be involved in endocarditis (inflammation of the heart valves), chronic lung infections, dental plaque, chronic wounds, infections on orthopaedic prostheses, ear tubes, vascular prostheses and various other conditions.
Although they are common, biofilm infections probably get relatively little attention because of their enormous diversity. They are extremely widespread, which contributes to the frequency of biofilm infections not being seriously recognised. They are everywhere but do not dominate numerically in each individual medical specialty apart from dentistry.
Nevertheless, biofilms result in considerable tolerance to antibiotic therapy and treatment failure if the treatment is not tailored to the biofilm properties of the microorganisms. Unfortunately, these are still not at all well known enough.
Young and unknown
Antonie van Leeuwenhoek observed and described more than three centuries ago how microorganisms deep inside tooth scrapings survived, whereas superficial microorganisms died when exposed to disinfectants. Nevertheless, biofilm infections in the rest of the body still comprise a relatively young research discipline that was first seriously presented in clinical practice 40–45 years ago. Natural and environmental microbiologists, in contrast, have known this concept for almost a century, and the technical sciences were interested in microbial biofilms very early.
With increasing clinical recognition of the importance of biofilm infections, the vast knowledge created in technical scientific settings needs to be used and hypotheses tested based on clinical observations and knowledge in model systems – both in the laboratory and in living organisms.
But the distance between the sciences is considerable. Very few scientifically active clinicians and basic researchers work across this gap, and useful knowledge is lost because of insufficient research in this field – both hypothesis-based basic science and applied translational research.
The missing solutions must be found and tested in this area and will therefore significantly benefit from improving clinically oriented scientific opportunities across the many types of biofilm to consider the diversity of the causes of biofilms: the various bacteria or fungi that attack organs or foreign bodies such as prostheses.
Together they become strong
The commonly known way microorganisms grow is individual planktonic growth, such as in laboratory flasks with liquid culture media. Biofilms, in contrast, are larger aggregates of bacteria or fungi organised in and surrounded by a self-produced polymer matrix.
The aggregates comprise long chains of various sugars – exopolysaccharides – extracellular DNA and other products such as lipopolysaccharide, proteins and peptidoglycan, which are secreted when bacteria die. The matrix may also contain constituents from the environment, including host factors in clinical biofilms.
The biofilms have significant gradients from the surface (with the same conditions as the immediate surroundings) and towards the centre of the biofilm, where the oxygen concentration can be very low and substrates can be limited. The biofilms either adhere to artificial or tissue surfaces or arise in tissues or tissue secretions.
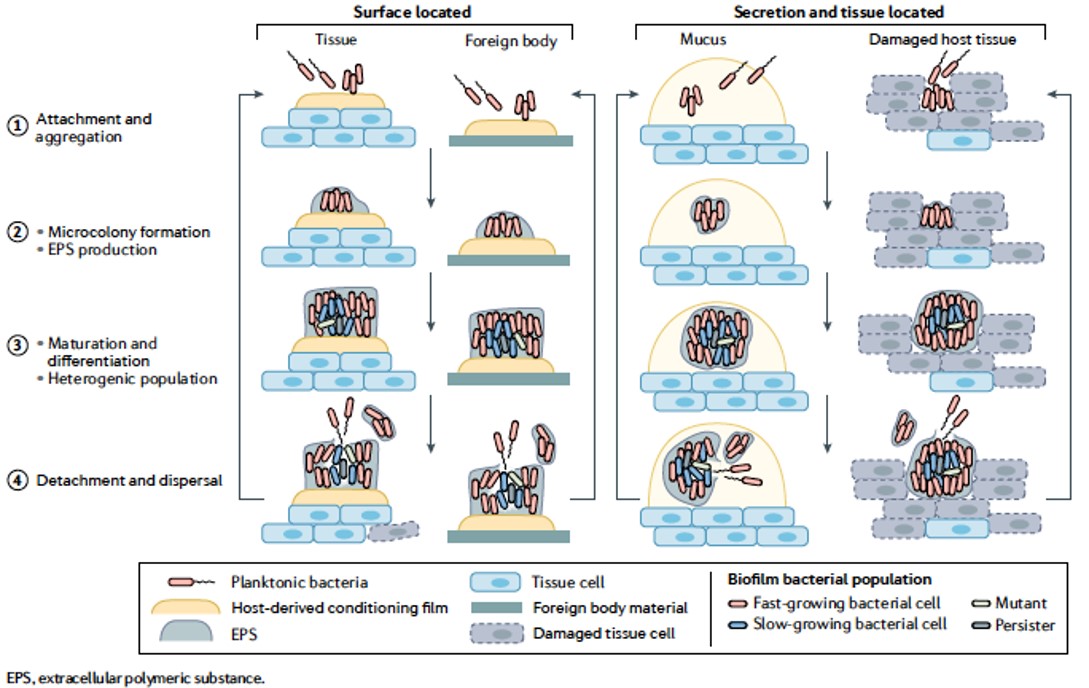
Biofilms are an organized form (aggregates) of a bacterial population (single or multispecies) with qualitatively different properties compared with a planktonic population of similar size. (From Nature Reviews Microbiology)
When tolerance is not good
The most serious consequence of biofilm in the body is the accompanying physiological tolerance to antibiotics, the immune system and disinfectants. The resulting tolerance is often not marginal but requires that the amount of antibiotics be increased to high concentrations to eliminate the microorganisms growing in biofilms.
Systemic drug treatment – throughout the body rather than locally – would be toxic at those concentrations.
Further, the immune response – effector functions such as leukocytes, antibodies and the complement system – damages the surrounding tissue rather than eliminating the biofilm and can lead to reduced organ function.
This collateral injury can lead to tooth loss in periodontitis, loosening of hip and knee prostheses, lung damage among infected people with cystic fibrosis, failure to heal among people with chronic leg ulcers and diabetic foot ulcers and destruction of the heart valves among people with endocarditis (heart valve infection).
Biofilms sleepwalk to success
The extreme drug resistance of biofilms can be considered an ancient survival strategy for microorganisms, and it is far more universal than, for example, spore formation, which is only found in very few pathogenic bacteria that infect people. All bacteria and fungi probably achieve tolerance by forming biofilms except the obligate intracellular bacteria.
The mechanisms behind the development of tolerance are multifactorial, but the reduced microbial activity in the central parts of the biofilms is perhaps most important, resulting in dormant microorganisms.
Most antibiotics depend on the microorganisms being active for optimal effect. Dormant biofilm bacteria that reduce their growth to one tenth (or lower) of their normal growth rate require 10 times longer treatment to be eliminated, and this is difficult to carry out. Sleep thus forms the very foundation of success for biofilms.
Translational clinical success
The first time a biofilm infection was clinically detected was in chronic lung infections – with Pseudomonas aeruginosa – among people with cystic fibrosis, a hereditary disease. They are therefore today considered a model for and have formed the basis and inspiration for studies of several other chronic infections and new treatment options:
- combination treatments for improving antibacterial effectiveness and preventing the development of drug resistance;
- antibiotic inhalation therapy to achieve high concentrations in the upper respiratory tract;
- subinhibitory effects affecting the virulence of the bacteria;
- maintenance treatment to suppress the ongoing biofilm infection; and
- DNAse treatment that can degrade DNA from white blood cells in mucus and extracellular DNA in the biofilm matrix.
And what has led to this success? The translational and interdisciplinary approach – the close collaboration with paediatricians and clinical microbiologists and, later, infectious disease physicians and ear, nose and throat specialists. But another factor is the close collaboration with the University of Copenhagen and the technical sciences, especially the Technical University of Denmark, which has used various advanced technologies to provide insight into development stages and niches in biofilm and the molecular background for observations.
Relevant model systems in animals for testing hypotheses have been a big part of the explanation – in vivo veritas! The personal relationships in the collaboration and the constant inventory and publication of the experiences have also been important.
Wound healing and endocarditis
Based on the observations of others but also based on our results using an animal model and an in vitro simulation model, we have shown that endocarditis is a biofilm infection.
By applying the biofilm principle of combination therapy, we helped to show that people with endocarditis can switch to tablet antibiotic therapy and return home from the hospital on average 16 days earlier, with even better long-term survival than intravenous inpatient antibiotic therapy.
In animal experiments, we have shown that hyperbaric oxygen therapy improved the elimination of bacteria on the heart valves and reduced inflammation. This resulted in a clinical study of hyperbaric oxygen therapy of people with endocarditis – a translational and interdisciplinary collaboration with the departments of cardiology at Herlev & Gentofte Hospital and Rigshospitalet and the hyperbaric oxygen therapy unit at Rigshospitalet. The results are currently being analysed.
Endocarditis results from endothelial damage to the heart valves that initiates a healing process through platelets, fibrin and leukocytes. But the endothelial damage also makes the heart valve more prone to bacterial colonisation.
This blood composition can also be achieved by using biological patches created from blood through a special form of centrifugation. Placing these autologous patches in chronic wounds has been shown to promote healing. In vitro colonisation of the patch with relevant endocarditis bacteria has been shown to work convincingly as an endocarditis simulation model and must now be tested to determine whether it can reflect the individual disease trajectory by testing treatment on a simulation based on the patient’s own blood and infecting bacteria.
The model should be used in parallel to identify new and improved treatments for people with endocarditis.
If it works in one place, it might also work in …
Immunoglobulin Y (IgY) is the most important antibody for birds. It is present in high concentration in the egg yolk (Y for yolk), and open studies have shown that people with cystic fibrosis who gargle with the yolk of chickens vaccinated against P. aeruginosa can prevent lung infections with P. aeruginosa from flaring up.
We have found even better effectiveness of IgY by combining it with azithromycin, an antibiotic. Such a strategy in prophylactic cystic fibrosis treatment has now been superseded by the new and extremely promising cystic fibrosis transmembrane conductance regulator (CFTR) modulators and potentiators. However, the combination may now be used to treat people with chronic obstructive pulmonary disease and bronchiectasis.
What works against one biofilm can apparently also be used against others based on the observation that treatments that work on one mucosal membrane may also work on another. We have just demonstrated prophylactic effectiveness of anti-Pseudomonas IgY on chronic urinary tract infection in a newly developed animal model. The clinical target groups are people for whom urinary tract infections are a widespread problem. This is especially relevant for people with spinal cord injuries who depend on catheterising the urinary tract several times a day, but people in intensive care may also be candidates for this strategy.
P. aeruginosa is obvious as a target bacterium because of its biofilm abilities, generally reduced antibiotic sensitivity and widespread ability to develop drug resistance, but IgY treatment can also relatively easily target other urinary pathogens; chickens can be easily vaccinated with other urinary pathogens.

A translational no-man’s land
To put it bluntly, clinically oriented translational research can end in the gap between patient management and basic scientific and technical research: where the complexity of animal models and how much work lies in ensuring their relevance and reproducibility are not well enough understood. Basic research has natural and limited insight into the complexity of patient treatment and of clinical trials of new treatment options.
It is almost a no-man’s land, but close intersectoral collaboration and understanding of the complexity and diversity of biofilm infections can make completely new and necessary clinical advances, also for various types of biofilms, to benefit patients.
The enormous success of cystic fibrosis treatment in Denmark has been a locomotive for the whole world, and since Denmark has very strong scientific biofilm-focused research communities with international collaboration, we should be able to make considerable progress within several other biofilm-related diseases and thus also involve others.
We are on track for chronic wounds, urinary tract infections and endocarditis, but the potential is far, far greater. So we are not home yet – we must move on!
