When the world was locked down because of the COVID-19 pandemic, a miracle was needed to reopen it. Vaccine researchers around the world dropped what they were doing to join the collective race against the new virus. The first to reach the finish line was considered a real outsider: the mRNA vaccine. However, many people do not know that the work on mRNA as a new drug class started decades ago. The four recipients of the 2022 Novo Nordisk Prize tell the story behind the development: the failures, the persistence and the distrust in the technology they are now certain will also revolutionise the approach to treating many otherwise severe diseases.
Although it may seem like forever, only 2 years have elapsed since the initial reports of a novel virus from Wuhan Province in China reached the global media. Physician and scientist Uğur Şahin, CEO of the German biotech company BioNTech, followed the reports through The Lancet, which published an article on the first cases of SARS-CoV-2 infection. What he was reading would eventually turn the world we know upside down.
“There were people who had no symptoms, no fever, even though they tested positive for SARS-CoV-2. I saw the virus as a cancer that was already metastasising unnoticed. Only later do you see what happens. Similarly, I saw planes taking off from Wuhan filled with apparently healthy people who only later became ill and began to infect others. I very roughly calculated that the virus that caused the outbreak had already spread all over the world. I was sure: we had to act immediately,” explains Uğur Şahin.
Speed created distrust
Together with his wife, co-founder and Chief Medical Officer of BioNTech, Özlem Türeci, Uğur Şahin decided to pivot a significant part of the company’s resources from their ongoing development of a cancer vaccine to develop a vaccine against the virus as rapidly as possible.
“We called it Project Lightspeed – to make clear that the only limitations that will not be questioned are the ones of physics; everything else will be challenged by science time,” explains Özlem Türeci.
However, of the 178 vaccine candidates that global researchers have developed against COVID-19, very few people thought that the Lightspeed Project would win the race. The announcement in November 2020 that BioNTech and Pfizer’s mRNA (messenger RNA) vaccine was 95% effective against COVID-19 was the beginning of the end of the pandemic. The vaccine created faith that there was light at the end of the tunnel, but it also created distrust of how vaccine development, which usually takes years, could now be achieved in less than one year.
“What many people around the world did not know was that scientists around the world have been working tirelessly to understand and optimise this tiny molecule. A molecule that many considered unpromising, because it is too unstable, too weak in protein production and inflammatory,” says Özlem Türeci.
“We saw the beauty of mRNA and have spent decades to discover ways to overcome its weaknesses. When the pandemic hit, we believed that this technology was one the best bet to beat the pandemic in time,” explains Uğur Şahin.
The stress of life
The success of the mRNA vaccines was also no surprise for two other pioneers behind the development of mRNA vaccines: Hungarian biochemist Katalin Karikó, senior vice president at BioNTech and an adjunct professor of Neurosurgery at the University of Pennsylvania and her United States colleague Drew Weissman, Professor in Vaccine Research at University of Pennsylvania, whose research achievements were a key part of the foundation for Project Lightspeed, which they started three decades before COVID-19 struck.
“When I started my career, we did not think about stopping pandemics. We were thinking about making new vaccines. Before COVID-19 struck, we had five Phase 1 clinical trials with different mRNA vaccines on which we were working. And we knew at the time that this technology had the potential to become an incredible vaccine, but to see this phenomenal success is naturally overwhelming,” explains Drew Weissman.
“I had never dreamed that my research would have that effect on anyone,” says Katalin Karikó.
It was not at all obvious that Katalin Karikó would become a researcher at all. As the daughter of a butcher and an accountant, she grew up in the 1950s and 1960s in the small town of Kisújszállás, Hungary. The natural sciences fascinated her already in school, and The Stress of Life, a book by Hungarian-Canadian endocrinologist Hans Selye, especially inspired her.
In particular, his thesis that the right attitude can transform negative stress into positive stress proved to be very important for her further career.
“As I continued as a researcher, I remembered Selye’s words about failure and the right attitude. When one door closes, another one opens. I would not be where I am today without all the rejections and demotions I have received during my career. So I do not get angry at these people who tried to stop me. If you are pushed aside, you are pushed aside. But you just have to keep pursuing your dream,” recalls Katalin Karikó.
The lesser-known cousin
Katalin Karikó has encountered plenty of opposition on her journey. Her research career began at the Biological Research Center, in Szeged, Hungary, where she originally tried to get cells to take up liposomes – small pieces of genetic DNA encapsulated in small spherical structures – bounded by a membrane comprising fatty acids.
“The idea was to get the body to produce its own medicine to combat many diseases by using DNA templates that was transported in with the liposomes,” explains Katalin Karikó.
However, DNA was later replaced by another nucleic acid molecule – its then-lesser-known cousin, RNA. Both molecules are built up by long chains of four nucleotide bases: adenosine (A), cytidine (C) and guanosine (G) and then thymine (T) in DNA and uridine (U) in RNA.
“The sequence of the nucleotide letters defines the unique genetic code for all organisms. In cells, genes – made from DNA – reside in the nucleus. When these DNA originals are to be translated into proteins, small mRNA copies of the respective DNA originals are made,” adds Katalin Karikó.
The RNA copies then move out of the nucleus, where they are translated into the body’s building blocks: proteins.
“One advantage of using RNA instead of DNA is that you do not have to get the RNA all the way into the cell nucleus and you do not mess with the originals – the genes,” says Katalin Karikó.
The last money stuffed in a teddy bear
When she completed her PhD degree, Katalin Karikó continued as a postdoctoral fellow in Hungary, but in 1985 the laboratory lost its funding. She applied for jobs in the United Kingdom, Spain and France, but no one wanted to hire her since she could not bring money from Hungary with her for her research. She finally got an offer from Robert Suhadolnik at Temple University in Philadelphia.
“I had to leave Hungary with my husband and my 2-year-old daughter. The laws were such that we could not leave with more than USD 100 in cash, so we hid our last money in her teddy bear – about USD 1200, which we got by selling our car and exchanging the money on the black market,” recalls Katalin.
Robert Suhadolnik was interested in a small molecule comprising three identical nucleotides. It was called 2′,5′-oligoadenylate (2-5A), and Katalin Karikó had worked with it in Hungary. Evidence indicated that 2-5A could initiate an immune response when double-stranded RNA is delivered into the cells in the hope that this would create an antiviral response to the global epidemic of the 1980s: HIV.
“These were the early clinical trials with double-stranded RNA, and we published the findings in a prestigious journal, The Lancet,in 1987, but unfortunately it turned out that such therapy did not help people infected with HIV,” says Katalin Karikó.

Katalin Karikó with her husband Béla Francia and their 2.5 years old daughter Susan in September 1985 at their first car a month after their arrival to the USA.
Nobody wanted to provide support
Many others tried and many failed. Philip Felgner’s laboratory at the University of Wisconsin–Madison in the United States got protein expressed in 1990 by injecting RNA and DNA into muscle cells of mice. In 1993, Martinon, Meulien and colleagues in France encapsulated mRNA in liposomes and injected them into mice, eliciting an immune response against the influenza virus protein encoded by the mRNA.
“The problem, however, was the stability of the RNA molecules. In the laboratory, they were frequently broken down by other enzymes, ribonucleases, and no one could figure out how it would ever become possible to store RNA as a medicine on a shelf either. In addition, we had no idea how to get the RNA to the right cell and to get the cell to produce enough protein from the RNA,” explains Katalin Karikó.
For the next 10 years, Katalin Karikó lived a turbulent research life. She served as a postdoctoral fellow at Uniformed Services University of the Health Sciences in Bethesda, Maryland and then as a research assistant professor at the University of Pennsylvania in Philadelphia.
“Just as I thought I was on my way to becoming a full professor, my applications for grants were rejected and instead I was demoted by the University in 1995. Nobody really believed that RNA would ever work as a therapeutic molecule,” says Katalin Karikó.
Photocopier meeting
There were only a few people along the way who recognised Katalin Karikó’s vision and hard work and therefore advocated on her behalf. Elliot Barnathan, a cardiologist, continued to believe in Katalin Karikó’s ideas and kept her on the payroll for his research on improving blood vessel transplants. When he left the University of Pennsylvania, neurosurgeon David Langer persuaded a department chair that they needed a molecular biologist.
Katalin Karikó’s career continued on borrowed time until one day in 1998 when she passed a new colleague at the department’s photocopier. Before scientific journals went online, researchers had to copy articles to archive them and keep up with the latest developments.
“I saw this new guy at the photocopier. He told me that he had only recently arrived at the University of Pennsylvania and wanted to develop an HIV vaccine that might be able to cure HIV or prevent HIV transmission,” recalls Katalin Karikó.
The two researchers soon realised that they shared a special interest.
“I had always wanted to test mRNA for my vaccines,” explains Drew Weissman, “and suddenly someone at the photocopier told me that she was doing exactly that. This was the start of a fantastic collaboration that has lasted ever since.”

Katalin Karikó and Drew Weissman with his students and technicians celebrating acceptance of David Scale (second from right) to Yale’s MD PhD program.
Dedication and passion
In addition to RNA, Katalin Karikó and Drew Weissman shared their enormous dedication and passion for science, a passion that Drew Weissman has had since childhood. This dedication earned him an MSc in Biochemistry in record time from Brandeis University in 1981.
He trained as a doctor at Boston University School of Medicine because he thought it would make him an even better scientist. He soon received a scholarship from the United States National Institutes of Health, led by the current director of the National Institute of Allergy and Infectious Diseases, Anthony Fauci.
“The Internet did not yet exist, so people from all over the world sent questions to the laboratory about diseases and diagnoses,” says Drew Weissman.
Working in Anthony Fauci’s laboratory introduced him to a whole new understanding of the connection between working in a laboratory and the health of real people.
“I was fascinated by the immune system and especially the recent discovery of dendritic cells – which travel around the body and branch out like trees with tentacles that can stretch and retreat again, in constant search of foreign organisms.”
The dendritic cells seemed to be the key to how the immune system could learn to conquer pathogens.
“Besides being enormously dynamic, these cells are the most potent antigen-presenting cells in the immune system. They capture foreign objects in the body and present parts of the foreign matter on the surface of the cells, communicating to the other immune cells, such as the T cells, what they need to destroy,” explains Drew Weissman.
Fiasco with potential
Since the dendritic cells are the primary antigen-presenting cells, they are also the key to effective vaccination. Katalin Karikó did not know much about dendritic cells when she met Drew Weissman. But she could help him test the mRNA in the dendritic cells. Katalin Karikó brought her synthetic mRNA to his laboratory, and Drew Weissman injected this into mice.
“We had coded our mRNA molecules so that they would produce one of the key targets to fight HIV – a polyprotein called Gag that contains all key HIV proteins. Then we inserted them into the dendritic cells and finally observed what happened when the dendritic cells were injected into a mouse. The results were very positive, because we got a very strong immune response,” says Drew Weissman.
However, Katalin Karikó and Drew Weissman reacted somewhat differently to the results. In addition to activating the immune system’s T cells, the mRNA independently turbocharged the immune system.
“The results were unexpected and disappointing. The delivered mRNA elicited a strong inflammatory immune response in human immune cells,” recalls Katalin Karikó.
“Katalin was disappointed because this meant that mRNA could not be used as a pure therapy, instructing a cell to produce a protein the body needs. Conversely, I could see enormous potential, because mRNA sparked an extraordinarily strong immune response, which is desired in developing a vaccine, so when Katalin saw a fiasco, I saw potential,” explains Drew Weissman.
Under the immune system’s radar
Katalin Karikó and Drew Weissman spent the next few years investigating the cause of the immune response and trying to modify the artificial RNA so that – if necessary – the natural immune response to injected artificially produced mRNA could be attenuated.
“We tested the immune response to various types of RNA molecules and found that transfer RNA (tRNA) molecules – in contrast to mRNA – did not induce any immune response,“ says Drew Weissman.
Similar to DNA, RNA comprises rows of four nucleosides – adenosine (A), cytidine (C), guanosine (G) and uridine (U) – but in tRNA molecules, several uridines are modified into pseudouridines.
“When we made the same change in mRNA, we suddenly saw the same effect. The molecules flew under the radar of the immune system so they could enter into the cells without alerting the body’s defences. The molecules changed from being lethal to the mice to being harmless,” recalls Drew Weissman.
A door closes
The pathway was now finally clear for Drew Weissman and Katalin Karikó to throw themselves into developing both mRNA vaccines and treatments for such diseases as haemophilia and Fabry disease, in which a missing or defective protein needs to be replaced. They therefore created spin-out company RNARx to develop mRNA to treat anaemia.
Katalin Karikó and Drew Weissman further improved their technology so that, in 2012, they used mRNA to get mice to increase their production of erythropoietin (EPO).
“We chose EPO-encoding mRNA, since demonstrating the effect of EPO on red blood cell production was straightforward. With a single injection of 100 nanograms of EPO-encoding mRNA, the amount of EPO increased markedly after just 6 hours and remained high for 4 days. So we thought that it had potential for clinical application,” says Katalin Karikó.
However, Katalin Karikó and Drew Weissman ended up not being able to get the licence for their company.
“The University ended up selling exclusive patent rights to a laboratory reagent supplier, CELLSCRIPT, for USD 300,000. That was the beginning of the end for RNARx, which we had to close in 2013, but as usual, when one door closes, another one generally opens,” says Drew Weissman.
Same problem – different solution
A new door opened at the headquarters in Mainz where another research group had been working on mRNA and immunotherapies for more than 20 years. Their paths crossed back in 2013 when Uğur Şahin, CEO of BioNTech, invited Katalin Karikó to give a lecture in Mainz, Germany on her technology for producing non-inflammatory mRNA.
“I was interested in their technique because they had found entirely different ways to address the same weaknesses of mRNA of not being sufficient potent,” explains Uğur Şahin.
Despite being on different sides of the Atlantic and not being in direct collaboration or contact, the researchers seemed to be on the way to solving the same issues at the same time – in different ways.
“Our solution did not clarify the inflammatory potential of mRNA, which we think is critical for a cancer vaccine. I was curious to learn more about the non-inflammatory mRNA approach and to see whether we could work together to further optimise mRNA. I therefore invited Katalin to give a lecture with us in Mainz and fortunately started collaborating,” says Uğur Şahin.
“Uğur and Özlem knew my work around RNA, and I knew their work. My father was a butcher, and Uğur’s father was a factory worker. The mRNA community was a small one back in these days and we knew each other’s publications well. In addition, we were all immigrants and worked on the same unpopular molecule: mRNA. We hit it off right away,” explains Katalin Karikó.

Ugur Sahin and Katalin Karikó first met in 2013: The mRNA community was small and well connected. Interested in Karikós and Weissmann's scientific work, Sahin invited Karikó to give a lecture at the University Medical Center of the Johannes Gutenberg-University Mainz, Germany.
A bit like in a Hollywood movie
Uğur Şahin’s family is from Turkey but moved to Germany in 1969 when Uğur Şahin was 4 years old. He grew up in Cologne, Germany and studied medicine at the University of Cologne.
“I was passionate about mathematics and science as a young boy. I was in the Catholic church library every weekend. The librarian put new scientific books and popular science journals aside for me,” says Uğur Şahin.
His interest in fighting cancer through the body’s immune system was sparked as a high school student when a relative developed cancer.
“After graduating, I was hired as a doctor to treat cancer patients at the Saarland University Medical Center, and that is where I met Özlem,” recalls Uğur Şahin.
Özlem Türeci grew up in Germany as the daughter of a biologist and a doctor who came from Turkey. However, it was the nuns at her father’s hospital who inspired Özlem Türeci.
“I was six or seven years old when my father allowed me to watch the first surgery in my life. I knew that I wanted to do the same,” Özlem Türeci recalls.
Like him and the nuns, she wanted to help people in need and therefore decided to study medicine at the University of Saarland. The two ended up working in the same hospital ward, and they both spent their spare time in the lab researching new ways to harness the immune system against cancer.
“Uğur and I both treated people with cancer. We are also both immunologists and met in the hospital during our shift. It was a bit like in a Hollywood movie. We fell in love with mRNA and each other,” Özlem Türeci adds
Cancer treatment of the future
During the day, they worked closely to treat cancer patients as good as they could and discussed the medical needs of the patients.
“We asked the question early on: how can we as doctors fulfil medical needs when the current standard of care for people with cancer cannot? Early on, we therefore started to work in the labs in the evenings and on the weekends to find better ways to treat cancer,” explains Özlem Türeci.
Uğur Şahin and Özlem Türeci’s path to mRNA therapies began in cancer research in the mid-1990s. They focused on developing cancer vaccines, including truly individualised ones, to present a patient’s immune system with the antigens of the patient’s own tumour to stimulate target-specific destruction.
“We knew that each patient’s tumour is unique, which led us to the conclusion that every treatment should be tailored to this patient’s tumour,” says Uğur.
“We focused on the idea that activating the immune system – through a vaccine – could create the cancer treatment of the future,” adds Özlem Türeci.
Personalised vaccines
To develop a new cancer treatment addressing this issue, Uğur Şahin and Özlem Türeci faced three major challenges that were considered insurmountable at the time.
“First, mRNA was still not potent enough to be used against many diseases such as cancer. Even quite small tumours consist of billions of cancer cells. To combat this preponderance of cancer cells, the vaccine had to be extraordinarily effective and able to generate billions of immune cells,” explains Özlem Türeci.
Further, the tumour characteristics that are recognised by the immune system are different for each and every individual patient. Uğur Şahin and Özlem Türeci recognised that an individualised vaccine technology was needed to tailor vaccines to each patient’s antigen profile.
“Most importantly, individualised vaccines had to be produced very quickly so that they could be administered in a timely manner to patients awaiting treatment before the cancer spread further,” says Özlem Türeci.
Entrepreneurs out of desperation
Cancer immunotherapy is the field in which research on mRNA-based technologies has been around the longest. As early as 1995, researchers from David Curiel’s group at the University of Alabama in Birmingham showed that injecting mRNA that encodes a tumour marker could elicit an antibody response.
“The mapping of target antigens to be chosen for a given immunotherapy showed that one target could not be identified for each type of cancer and that the same targets are not even found among different patients with the same type of cancer. That is how our vision of individualised cancer treatment emerged,” says Özlem Türeci.
However, gene sequencing was still slow and expensive in the 1990s, and like Katalin Karikó and Drew Weissman, Uğur Şahin and Özlem Türeci found that the early promising results with mRNA therapy did not lead to major funding or investments. Instead, in the years that followed, cancer researchers pursued therapeutic approaches based on DNA and proteins.
“We became entrepreneurs out of desperation because we learned that it takes much more than just the discovery to transform science into survival,” Özlem Türeci recalls, explaining why she first became a physician, then a scientist and then an entrepreneur in 2001.

In 1999, Ugur Sahin and Özlem Türeci were immunologists at the same hospital in Germany treating patients with cancer in their days and dong research in their evenings to find better ways to treat cancer. They fell in love with mRNA and each other.
Too early, too risky, too difficult
In 2001, Özlem Türeci and Uğur Şahin therefore co-founded their first unicorn, Ganymed Pharmaceuticals, together with their mentor Christoph Huber, which translated research into a new class of anticancer drugs they called ideal monoclonal antibodies that bind solely to the surface of the patient’s cancer cells.
Özlem Türeci became CEO and Uğur Şahin head of research. But even though Ganymed Pharmaceuticals became a scientifically huge success, they had to sell the company because they could not afford the large Phase 3 trial to develop the antibody to market approval.
“We had to learn that research alone could not reach patients. Large trials and funding are required to create and eventually roll out a new medicine so it can ideally reach millions or billions of people worldwide,” explains Özlem Türeci.
And access to funding for mRNA was not easy following the financial crisis in 2008.
“Too early, too risky, too difficult – those were the reactions we heard a lot. When we presented our vision to a biotech investor, his answer was that mRNA-based tailored vaccines are a good idea but will never work,” says Özlem Türeci.
Loud and clear
Luckily, Uğur and Özlem Türeci could convince a handful of private investors who believed in their vision since the very beginning. Among them were two German investors: the Strüngmann brothers, who founded Hexal and invested USD 180 million in BioNTech in 2007 following the sale of the company.
“They agreed to a no-questions-asked clause and to forfeit the right to force a sale for 15 years. This gave us breathing room to realise our vision and the goals we had set ourselves as young doctors 13 years previously,” recalls Özlem Türeci.
With the support Özlem Türeci and Uğur Şahin could now develop and produce individualised and innovative cancer immunotherapies including their dream of RNA vaccines. Already in the late 1990s, they had begun a systematic discovery and optimisation process to solve the basic problems of RNA: low and short-lived protein production.
“We decided to focus on the non-protein-coding structural components of mRNA, including the cap, which aids in exporting mRNA to the cell cytoplasm, and the poly-A tail, which assists in binding to the ribosome, where the translation into protein takes place,” explains Uğur Şahin.
Increased by several thousand times
Each of these components protects the mRNA from degradation and thus influences whether and how much mRNA is transcribed into protein, how long it is active and how quickly it is degraded. Uğur Şahin and Özlem Türeci discovered modifications in the cap, the poly-A tail and other untranslated regions of mRNA. The combination of modifications resulted in an exponential improvement in mRNA potency.
“We made these changes of the mRNA backbone not only to improve the stability of the molecule so that it did not break down as quickly but also to increase the quantity of protein produced inside the cell. We had thus found a way to communicate loud and clear with the immune system and tell it what to attack,” says Uğur Şahin.
Their breakthrough was published in the peer-reviewed journal Blood in 2006, followed by a series of articles in the years 2007 to 2010 describing additional improvements of the mRNA backbone.
“The combination of these optimised components increased the mRNA-induced antigen yield several thousand times, so this really made us believe that was now powerful enough to be used against both cancer and infectious diseases,” explains Uğur Şahin.
The systematic principles for mRNA vaccine design introduced by Uğur Şahin and Özlem Türeci became the widely used standard for optimising vector scaffolds for mRNA therapy. This was also the first step of developing the first mRNA-based vaccine in the history of medicine.
“In this way, a tiny amount of mRNA was able to induce immune responses strong enough to see tumours shrink in some of our patients in the clinical trials,” explains Özlem Türeci.
Uğur Şahin and Özlem Türeci started in 2010 to search for lipid formulations to help create small bubbles to protect the mRNA from degradation in the body and ensure that it was delivered to the lymph nodes and spleen. Meanwhile on the other side of the Atlantic, Drew Weissman, Katalin Karikó and research colleagues were independently testing nano-sized lipid bubbles as mRNA carriers.
Nano-sized bubbles
mRNA basic research had made great progress on both sides of the Atlantic. However, a significant problem still needed to be solved: the RNA had to be targeted to the right place. But what was the right place? In the vast majority of studies, mRNA was injected into the skin or into the muscle.
However, like Drew Weissman and Katalin Karikó, Uğur Şahin and Özlem Türeci believed that mRNA needs to reach the high-performance trainers of the immune system, the dendritic cells in the lymph nodes and spleen.
“Our first experiment, in which we injected mRNA into the lymph node, was a real game-changer. One morning, we were all sitting at the monitor connected to the immune cell–measuring device, and when we saw the strength of the immune response, we almost fell off our chairs,” says Uğur Şahin.
The mRNA vaccine injected into the lymph node had triggered an immune response that was dramatically stronger than the mRNA injected into the skin or muscle.
“We understood immediately that this was a crucial part of the solution. We had to find a way to get mRNA vaccine into the lymph nodes,” explains Uğur Şahin.
Act like a zip code
Uğur Şahin and Özlem Türeci started in 2010 to search for lipid formulations to help create small bubbles to protect the mRNA from degradation in the body and ensure that it was delivered to the lymph nodes and spleen.
“We used mRNA encoding proteins that emit light so we could study which organs lit up after administering mRNA in the different lipid formulations,” explains Uğur Şahin.
Two years later, Uğur Şahin and Özlem Türeci discovered a lipid nanoparticle formulation that acted like a zip code so the administered mRNA vaccine was delivered to dendritic cells in lymphatic tissues all over the body, where it could be translated into protein.
“This simultaneously allowed large numbers of dendritic cells to be targeted to generate a correspondingly large immune cell army that precisely recognised only the cancer cells. The results were impressive,” recalls Uğur Şahin.
But no one had previously studied nanoparticulate mRNA in humans. For 2 years, Uğur Şahin and Özlem Türeci’s team at BioNTech had to work hard to create the lipids in clinical grade and wait patiently for the results of a clinical trial among cancer patients that was published 2 years later in a seminal article in Nature.
“The clinical data really convinced us in the end. The treatment was well tolerated, and even patients treated with the lowest dose of just 14 µg showed an extremely strong immune response,” recalls Özlem Türeci.
A matter of balance
Meanwhile on the other side of the Atlantic, Drew Weissman, Katalin Karikó and research colleagues were independently testing nano-sized lipid bubbles as mRNA carriers in 2015. In collaboration with a research team at the University of British Columbia in Vancouver, Canada, they got the liver to produce a luminous protein for which their mRNA had coded – over a prolonged period of 10 days. Again, the United States and German teams – independently of each other – found different solutions to solve the same challenges.
“We developed methods for making the mRNA immunologically silent, whereas Uğur and Özlem used natural RNA to activate the immune responses that can boost the effect of a vaccine. Instead, we engineered the lipid bubbles so they could boost an immune response themselves. The most amazing thing with these nanolipids is that you decorate the surfaces slightly, so you can target them to specific places in the body,” explains Drew Weissman.
In 2015, after 30 years of research efforts, the four mRNA musketeers clearly understood that making RNA therapy work is a matter of balance.
“RNA being unstable is a problem, but the instability is also an advantage – as with other drugs – that they are broken down relatively quickly in the body, so they do not have a lasting effect. So, it is better to use many copies of an mRNA medicine that is broken down quickly than one copy of DNA that might alter the genome permanently,” adds Drew Weissman.
It is also a matter of balance concerning the immunogenicity.
“On the one hand, you need a vaccine that boosts the immune system. On the other hand, the immune response should not be too strong,” says Drew Weissman.

Drew Weissman and Katalin Karikó at the University of Pennsylvania in 2015.
Wanted – dead
Armed with this information, the researchers could now seriously dream of starting to treat and cure people with diseases that had previously been difficult if not impossible to treat. Already in 2014, Özlem Türeci and Uğur Şahin and colleagues treated their first patient with an on-demand manufactured mRNA vaccine that encoded the unique mutations in the tumour sample of that individual patient.
“When the dendritic cells take up the RNA, they produce small copies of the mutations, which are distinctive features of this patient’s cancer, that are sent to the surface of the cell and recognised by the immune cells. Thus, the body will know how to identify the cancer cells to be destroyed,” says Özlem Türeci.
She compares giving mRNA messages to the immune system to giving a sheriff a poster of a wanted villain. The portrait should be as accurate as possible. If the poster is inaccurate, the sheriff (the immune system) may not recognise the target, and even worse, attack and destroy the wrong target.
“In our first individualised mRNA cancer vaccine trial, we tailored mRNA vaccines for 13 people with metastatic melanoma, a type of cancer with a high frequency of mutations. We created a marked immune response for several months, so it made us seriously believe that our dream of developing personal cancer vaccines could succeed,” recalls Özlem Türeci.
Prophetic
The dream of mRNA vaccines in general further gained momentum in the following years. In 2017, the BioNTech co-founders and their team published study results in Nature about their successful development of individualised mRNA-based vaccines and the strong immune responses they were able to induce in cancer patients.
“We had now optimised the design and the manufacturing process of mRNA-based treatments so much, that we could now tailor a vaccine to a specific target within weeks,” explains Uğur Şahin.
The results were so encouraging that Uğur Şahin made a bold prediction at a conference in Berlin in 2018. In a room filled with experts on infectious diseases, he announced that his company might use mRNA technology to quickly develop a vaccine in the event of a global pandemic and that an mRNA vaccine could be adapted to regionally occurring variants in the case of a spreading infectious disease.
In the same year, Drew Weissman and colleagues, including Katalin Karikó, published a preclinical study in which they produced a vaccine against the Zika virus through mRNA formulated with lipid nanoparticles. In the same year, clinical studies of mRNA vaccines against rabies and influenza variant were initiated.

Ugur Sahin presenting the concept of individualized mRNA-based vaccines to tackle global health threats at the Grand Challenges Meeting in Berlin, Germany, in 2018.
Call again in a few weeks
Almost one year after Uğur Şahin’s statement, the first reports of a new coronavirus from China ticked in.
“The article in The Lancet on 24 January 2020 convinced us that a pandemic was on the way and that we should act, regardless of the risk to our company,” explains Uğur Şahin.
The article, written by researchers in China, provided the first strong evidence that what was later called SARS-CoV-2 could be transmitted between people, but something else sent a shiver down Uğur Şahin’s spine: a 7-year-old girl tested positive for SARS-CoV-2 without showing symptoms.
Uğur Şahin and Özlem Türeci persuaded the Supervisory Board of BioNTech that the company should react, and a few days after the genetic sequence of SARS-CoV-2 was published, BioNTech had charted a new course. However, BioNTech’s later partner, Pfizer, which was to manufacture any vaccine, was not convinced that it should join the project.
Pfizer’s Vice President and Chief Scientific Officer, Phil Dormitzer, initially rejected the offer to develop a COVID-19 vaccine because, like many others, he believed that SARS-CoV-2 would be contained, similar to the 2003 SARS pandemic and the MERS pandemic in 2012.
“After the phone call with Phil, I said to Özlem: ‘We will call him again in a few weeks,’” remembers Uğur Şahin.
At the speed of light
Uğur Şahin and Özlem Türeci persevered, and the next time Uğur Şahin called Pfizer, COVID-19 had started to cause many deaths in the United States, and Pfizer said yes. For the next 9 months, the researchers then applied more than 30 years of experience in developing mRNA vaccines in Project Lightspeed.
Uğur Şahin and Özlem Türeci created vaccine BNT162b2 by producing artificial mRNA and encapsulating it in lipid nanoparticles. When injected into the body, the tiny mRNA-filled nanoparticles travel toward the lymphatic tissues, where they are taken up by dendritic cells.
“The dendritic cells in the lymph nodes near the injection site take up the tiny particles and then produce copies of the SARS-CoV-2 spike protein and send the small posters of the villain to the surface of the cells. The dendritic cells act like generals and train specialised cells of the immune system to kill the villain,” explains Özlem Türeci.
But even though the researchers were confident the technology worked, many people were not convinced.
“Even though Moderna had carried out clinical trials proving the safety of the mRNA vaccine technology, we had to do our own safety studies before we could move into Phase 3 clinical studies involving humans. Our teams worked day and night; workstreams that normally occur sequentially had all been initiated in parallel,” recalls Özlem Türeci.
The day in November 2020, when the efficacy analysis of our Phase 3 study was performed, was the day of reckoning.
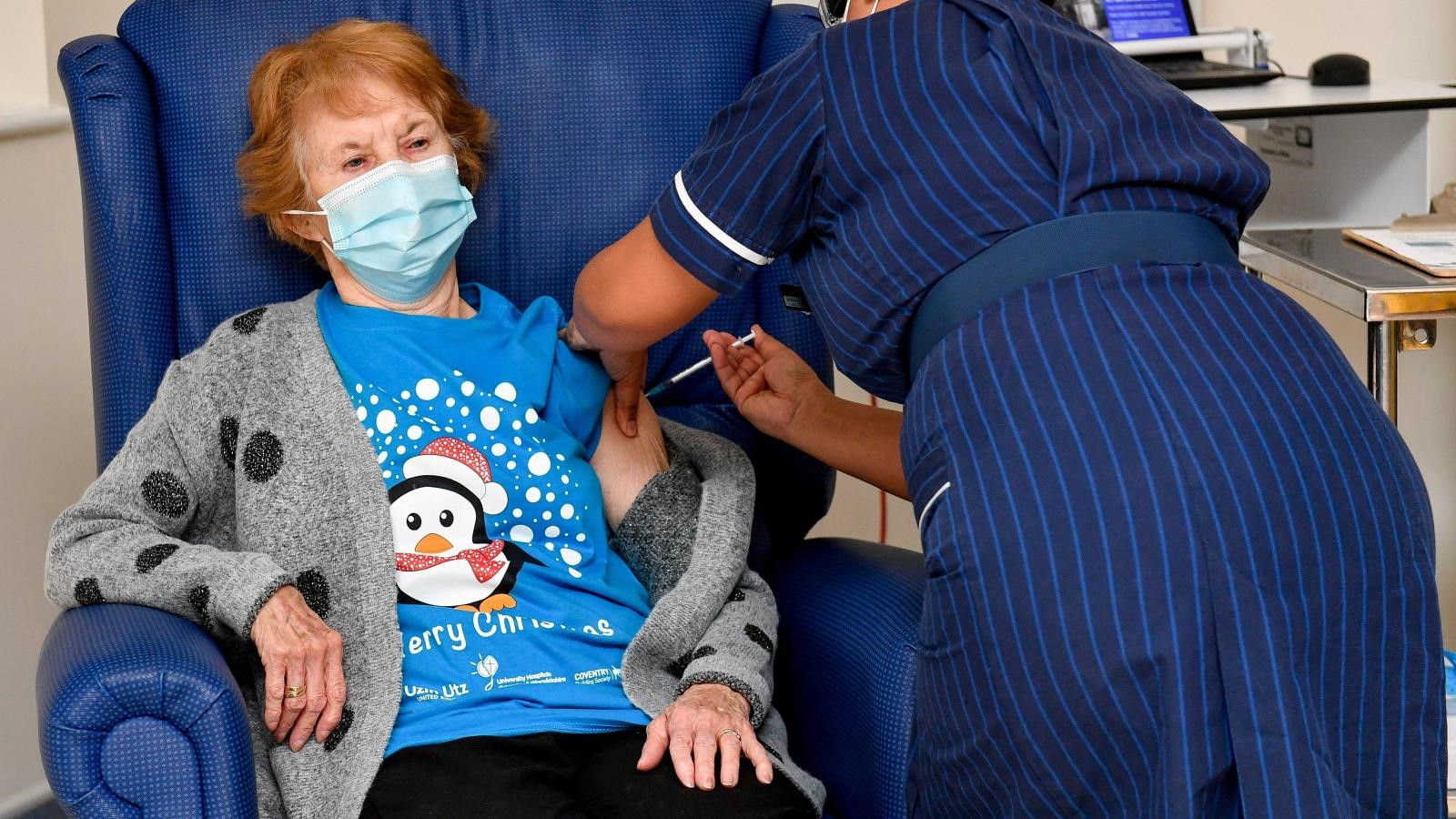
Margaret Keenan, 91, was the first person in the world to get a clinically-approved COVID-19 vaccine at University Hospital on 8 December 2020.
Deeply moved by the letters
On 9 December 2020, Uğur Şahin got a call from the CEO of Pfizer, Albert Bourla.
“‘Do you want to know the data?’, Albert asked. I said ‘No’. A really bad joke, and the next couple of seconds felt like an eternity, until he said ‘It works’,” recalls Uğur Şahin.
The results of the Phase 3 trials were made public the day after. The vaccine was 95% effective against COVID-19. Very few people had predicted that the mRNA vaccines from BioNTech/Pfizer and Moderna would be the first and most successful in curbing the COVID-19 pandemic. Neither the success nor the lack of faith in the success of the mRNA vaccines surprised the four researchers.
“Of course I was very happy, but I also expected it. Previous preclinical trials had already showed how effective these mRNA vaccines were against other infectious diseases such as influenza. I was still deeply moved by the letters people sent to me telling me what the vaccine meant to them. They could once again visit their beloved family,” says Katalin Karikó.
By the end of January 2022, 10 billion COVID-19 vaccinations had been carried out, and in November 2021, WHO estimated that the vaccines had directly saved 500,000 lives.
“This estimate does not even include lives saved from the indirect effect of vaccination reducing the infection rate so that the capacity of healthcare systems could keep up while waiting for a less lethal variant of SARS-CoV-2 to emerge,” adds Katalin Karikó.
A vaccine against the Omicron variant with the usual mRNA vaccine efficacy has already been manufactured and is being tested in clinical trials. It is about to be rolled out, just months after Omicron appeared. According to Özlem Türeci, it is still too early to determine when the battle against the pandemic will end.

Uğur Şahin, Özlem Türeci and Katalin Karikó anno 2022.
Democratising access to medicines
Katalin Karikó, Drew Weissman, Uğur Şahin and Özlem Türeci are well-known names in the scientific world and even famous in the public arena. All four have in common that obstacles to their careers have been commonplace and even a driving force, which fame has never been.
“By nature, scientists are doers and do not cry or complain. They do even more if the vision they are pursuing involves serving a greater cause,” says Özlem Türeci.
“We have created an mRNA toolbox and have learned how we can convey various messages to the immune system using mRNA. This is only one part. The other part is what content to communicate, and this requires deep mechanistic understanding of the diseases we want to target,” explains Uğur Şahin.
“Most of all, I am excited to join the further collaboration with research colleagues to explore everything else that mRNA vaccines can achieve. We are working on malaria vaccines for people in Africa and on leptospirosis vaccines for people in Southeast Asia. We are also working on vaccines against herpes, HIV and peanut allergy. Like the work with the COVID-19 vaccines, research is carried out through collaboration, so an incredible number of good people are behind this,” adds Drew Weissman.
“We believe that mRNA can democratise access to medicines. This fascinates me,” adds Uğur Şahin.
“I am extremely pleased that my research has helped to stop a pandemic, but I want to emphasise that many scientists and technical experts contributed to the success of the mRNA vaccines. Over the past few decades, they helped to advance the knowledge on various fields that built the foundation for our work. Few scientists are lucky enough to see people benefitting from their research,” says Katalin Karikó.
“Some of our friends call us immune engineers. We spent decades on basic research, and it is still at the core of our work. We believe that the journey is not over yet. It is just the beginning of a new era of medicine,” adds Özlem Türeci.
“The revolution in artificial intelligence, automatisation and robotics means that personalised vaccines for several types of tumours may start to become available within years. This will revolutionise cancer treatment. We think that this is possible within the next 5 years,” concludes Uğur Şahin with the same confidence and vision he displayed when launching the effort to develop the COVID-19 vaccine in January 2020.ne.
